Figure 1 shows the Jablonski diagram of singlet, triplet exciton states with a few vibrational sides. Light absorption with energy above the bandgap excites electrons from lower to higher energy levels. The excited charges tend to quickly relax back down to its lowest vibrational state and then decay to ground state either through the non-radiative or radiative channel. Fluorescence is a radiative relaxation process arising from charge relaxation from excited to ground state, also known as radiative recombination or radiative quenching.
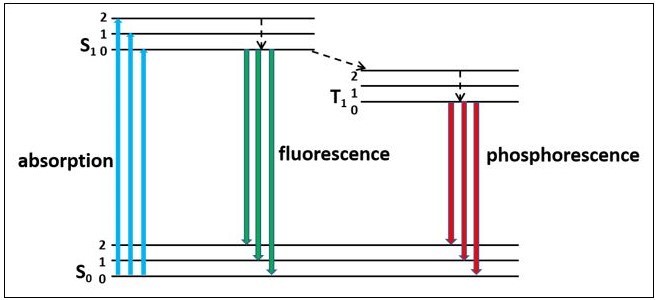
Figure 1. Jablonski diagram of singlet, triplet exciton states with a few vibrational sides.
According to Kasha’srule [1], once excited electrons energetically relax to the bottom of the excited state (mostly S1 state) within several femtoseconds or picoseconds, and emission occurs from the bottom of the S1 state to different vibrational levels in the ground state. Electrons in the excited state can be further promoted to higher excited states before relaxation, or they can undergo a non-radiative intersystem crossing process from S1 state to triplet state (T1). The relaxation from T1 state to the ground state (S0) (phosphorescence) is manifested at much longer lifetimes as compared to fluorescence, and it is a little red-shifted from fluorescence.
In general, the decay of excited states population is an exponential function and fluorescence intensity at any given time is proportional to the excited state population at the same time t. So, we can formulate fluorescence lifetime as F(t) α N*(t), where N*(t)= N*(0) Exp (-t/τ). Therefore, fluorescence lifetime can be simplified as:
F(t)= N*(0) Exp(-t/τ)
where N*(t) is proportional to the excited state population at the same time t and F(t) is fluorescence intensity at given time t, and τ is the average time that is spent by molecule in the excited states. If decay is not a single exponential like in the most scenario then fluorescence lifetime can be written as the sum of multiexponential with different amplitudes:
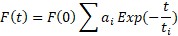
1- Kasha, M. Characterization of electronic transitions in complex molecules. Discus. Faraday Soci.9,14-19 (1950).