Microscopic is drastically changing in recent years. While there is a wide range of microscopic techniques available, you may need to consider what type of microscopic technique is more relevant to your biological problem. We provide a general guideline to choose your right microscopic technique.
Transmitted Light Microscope
Transmitted light is a very simple and non-perturbing technique for measuring cell behaviour. It doesn’t require any special preparation such as genetic encoding. Because transmitted light tends to be less damaging to the specimen than fluorescence excitation, it is an excellent technique for observation of cells behaviour without any photodamage.
Fluorescence Microscope
On the other hand, measuring a specific molecule in the cell or specific organelle require molecular marker and fluorescence microscope accordingly. Fluorescence microscopy is one of the most widely used imaging modality for biological research. It offers high contrast imaging and high specificity. It is also compatible with imaging live cells and offers monitoring living sample with relatively little perturbation. Having a reasonably fast time resolution also allow us to probe dynamics that are happening in the cell in real-time. Fluorescence microscopy is a very powerful tool to monitor and probe different gene products inside the cells simultaneously with high molecular specificity. However, the downside of fluorescence microscopy is a resolution which is limited to a few hundred nanometres.
There are many different types of fluorescence microscopic techniques available which based on the depth of penetration. To choose the right technique you need to consider what type of specimen you are going to study. Are you looking at a thick or thin specimen? The second question is that are you looking at living samples or fixed sample? While the time of acquisition and photodamage are not very important factors for fixed sample, for living cells we should consider how fast to observe dynamics in the cell without damaging the cell.
1-1 Epifluorescence (widefield)
The epifluorescence is a low-cost technique as it does not require laser as an excitation source. It can be used for imaging large quantity of fixed cells, over 10µm deep. However, due to out-of-focus fluorescence contribution in the plane of focus, there is a high background signal in the image. Producing a lot of haze in the image prevents us from getting a nice, crisp and clear image. Moreover, the epifluorescence penetrates through the entire cell and fluorescence excitation can potentially damage the cell.
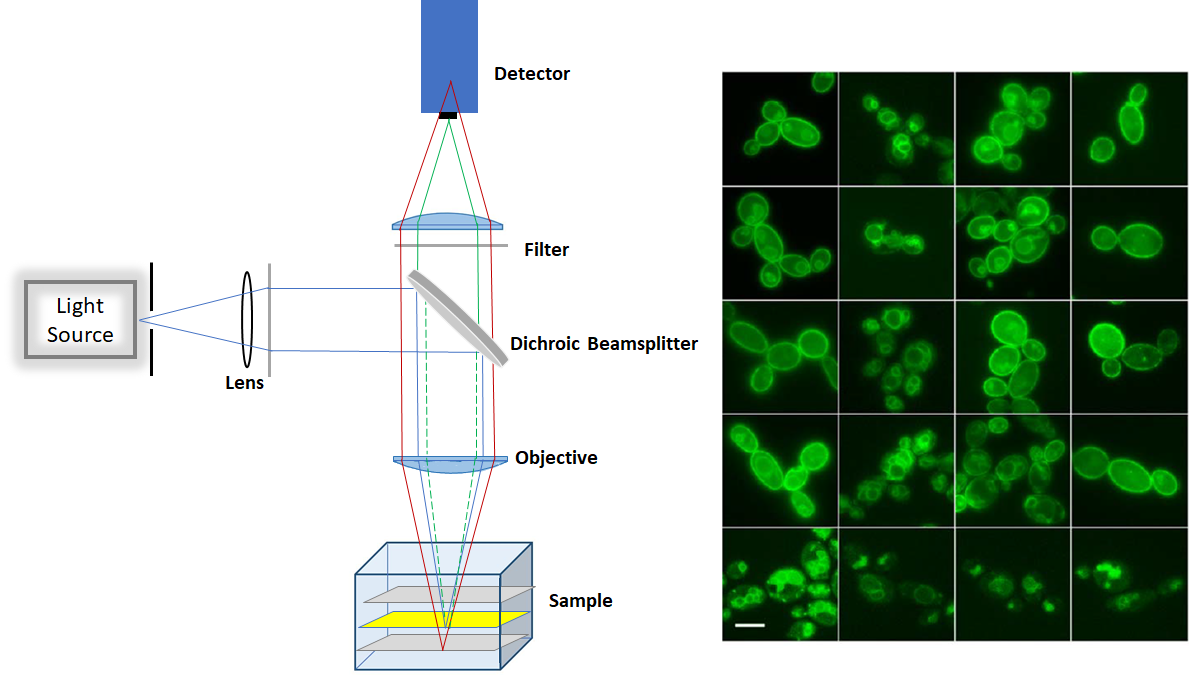
Figure1. Schemtaic of Epifluorescence microscop (left) and sample image (right)
1-2 Deconvolution Microscopy
Deconvolution microscopy is a computational technique that removes the contribution of out of focus fluorescence light. This technique requires an epifluorescence microscope and a motorized z-axis stepper to take images of different z sections through the specimen. Software is used to perform computational correction and sharpen in-focus light. Epifluorescence is very low-cost imaging technique as it does not require laser as an excitation source.
1-3 Array Tomography (physical sectioning and epifluorescence)
Physical sectioning is a common technique for creating the whole image of a piece of tissue, part of the brain or even the entire brain. In this technique, conventional epifluorescence microscopy is used to take the image of tissue that optically sectioned it into very small slivers. These slivers of tissues stain for various cellular makers. Then all these slices put together computationally to create the whole image of the tissue
2- laser-based sectioning microscopies
Depending upon the depth of the specimen, several laser-based sectioning microscopies are commercially available. In the following, we provided a brief summary of some of common techniques.
2-1 Total Internal Reflection Fluorescence
Total internal reflection fluorescence is a powerful technique for imaging specimen near the surface of the coverslip. It provides clear images with better signal to noise ratio compared to epifluorescence. It is a general tool for any type of biochemistry that can be visualized on a glass surface. For thicker sample like a cell or even a section of tissue, for example, < 100um, you may have to choose pin hole microscopy. Point scanning confocal microscopy, line-scanning confocal microscopy, and spinning disk microscopy are different types of pine hole microscopy.
2-2 Spinning Disk Microscopy:
Spinning disk confocal microscopy is a powerful technique for live-cell imaging and fast acquisition. It can be used for relatively thin sample less than 30um with little cellular damages.
2-3 Point Scanning Confocal Microscopy
For the slightly thicker specimen with less photodamage concern point scanning confocal microscopy is a better option. However, it tends to be slower than spinning disk and more suitable for fixed tissue. Because it focuses very intense laser at each point, it tends to be more photodamaging than spinning disk confocal. Point scanning confocal is a good technique for measuring dynamic of molecule in cells by combining photobleaching and imaging by the technique called recovery fluorescence after photobleaching.
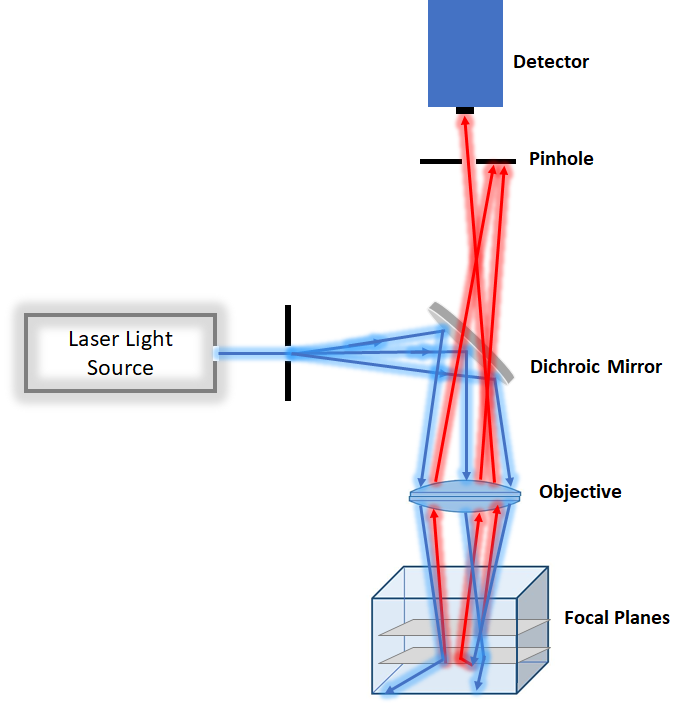
Figure2. Schemtaic of Confocal Microscopy
2-4 Light Sheet Microscopy
Light Sheet Microscopy is a very fast acquisition technique with low photodamage. This technique is commonly used for imaging embryos. In this technique, light travel perpendicular to the optical axis and thus only illuminate the specimen in one focal plane. So, excitation doesn’t damage above or below the field of observation. Due to little photodamage, it is a very good technique for imaging processes that occurs in a complex system such as embryos.
2-5 Two-Photon Microscopy
For thicker sample >100um, such as a piece of tissue, two-photon microscopy is the best choice.
2-6 Super-Resolution Microscopy
This method allows us to observe the interaction between molecules. So, we need a resolution that is up to the molecular scale of a few nanometres.